[ad_1]
In a new analysis, KFF finds that 3.6 million people with Medicare could be eligible for coverage of Wegovy (semaglutide) now that the Food and Drug Administration has approved the use of the anti-obesity drug to reduce the risk of heart attacks and stroke in certain patients.
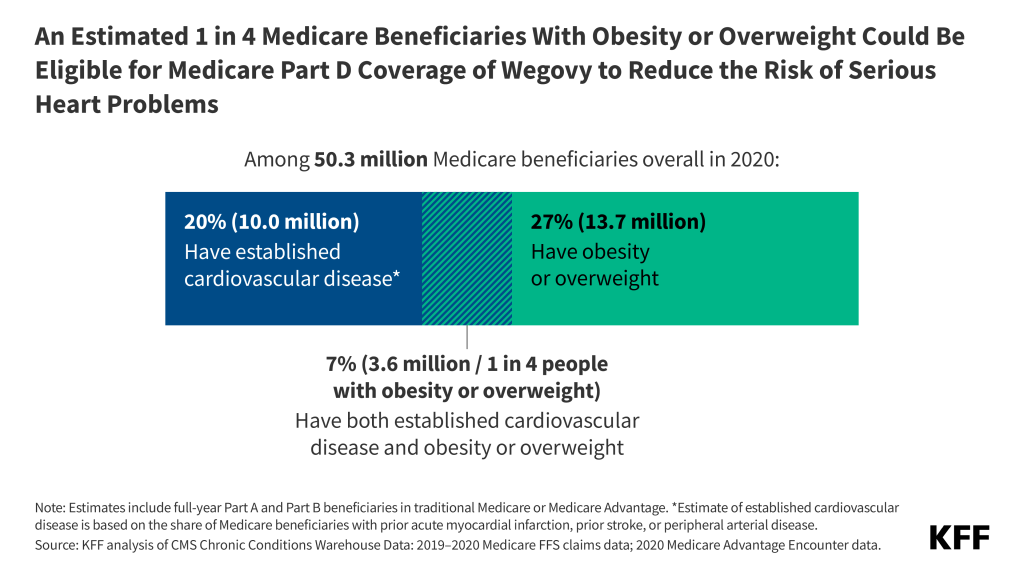
This change potentially allows access to Wegovy for just over 1 in 4 of the 13.7 million people on Medicare diagnosed with obesity or overweight, based on data from 2020, the analysis shows.
Based on KFF research, about 7% of beneficiaries, or 3.6 million people, had established cardiovascular disease and obesity or overweight in 2020 and could be eligible for coverage of Wegovy to reduce the risk of serious heart problems. (Among this group, 1.9 million also had diabetes, making them already eligible for Medicare coverage of other GLP-1 drugs approved for diabetes.)
Although Wegovy already had FDA approval as an anti-obesity medication, Medicare is prohibited by law from covering drugs when prescribed for obesity. The drug’s new approval by the FDA for use to reduce heart attacks and stroke paves the way for Medicare to cover it for those purposes.
How this change affects Medicare spending will depend in part on how many Part D plans add coverage for Wegovy and the extent to which plans apply restrictions on use like prior authorization, how many eligible people use the drug, and negotiated prices paid by plans. Assuming just 10% of eligible beneficiaries use Wegovy in a given year, and assuming a 50% rebate on the list price, Medicare would incur nearly $3 billion in additional net Part D spending for one year for this one drug alone.
KFF also finds that beneficiaries who take Wegovy could face monthly out-of-pocket costs of $325 to $430 if they have to pay a percentage of the drug’s $1,300 list price for a month’s supply. The new Part D cap on out-of-pocket spending would limit beneficiaries’ out-of-pocket cost to around $3,300 in 2024 and $2,000 in 2025—still significant sums for those who live on modest incomes.
It is possible that Medicare could select semaglutide for drug price negotiation as early as 2025, based on its earliest FDA approval in late 2017, with a negotiated price available beginning in 2027. This could help to lower Medicare spending on Wegovy as well as Ozempic, the version approved for type 2 diabetes. (Both the Part D out-of-pocket spending cap and Medicare drug price negotiations were established under the Inflation Reduction Act of 2022.)
The full analysis, and other data and analyses about Medicare spending on prescription drugs, is available at kff.org.
[ad_2]
Source link
